
Open Microperfusion OFM
Key words:
Classification:
- Product Description
-
BASI Open Microperfusion OFM - Advanced In Vivo Sampling Technology
Better data leads to better decisions
Our innovative OFM (open microperfusion) sampling technology is an invaluable tool for PK-PD studies in neuroscience, dermatology, oncology and biomarker research. The minimally invasive, membrane-free OFM probe enables the acquisition of full biochemical information of interstitial fluids from brain and peripheral tissues, opening new horizons for preclinical and clinical drug testing.



Effective
OFM studies reduce costs by providing a complete pharmacological profile early in drug development
Targets
The OFM probe is capable of continuous sampling from clearly specified tissue types
Universal
OFM samples all substances in the interstitial fluid, independent of size, lipophilicity or protein binding
Open flow microperfusion (OFM) technology is an in vivo sampling technique used to collect molecules at tissue-specific concentrations, regardless of molecular weight or lipophilicity.
- 1. It works on the principle of push-pull perfusion through a membrane-free probe.
- 2. The OFM probe has a macroscopic opening that allows the perfusate to mix directly with the surrounding interstitial fluid (ISF).
- 3. Sequential and repetitive sampling of bound and unbound drugs, neurotransmitters, antibodies, peptides, cells and other molecules.
- 4. OFM reduces costs and development time by providing pharmacological characterization of drugs in the early stages of drug development.
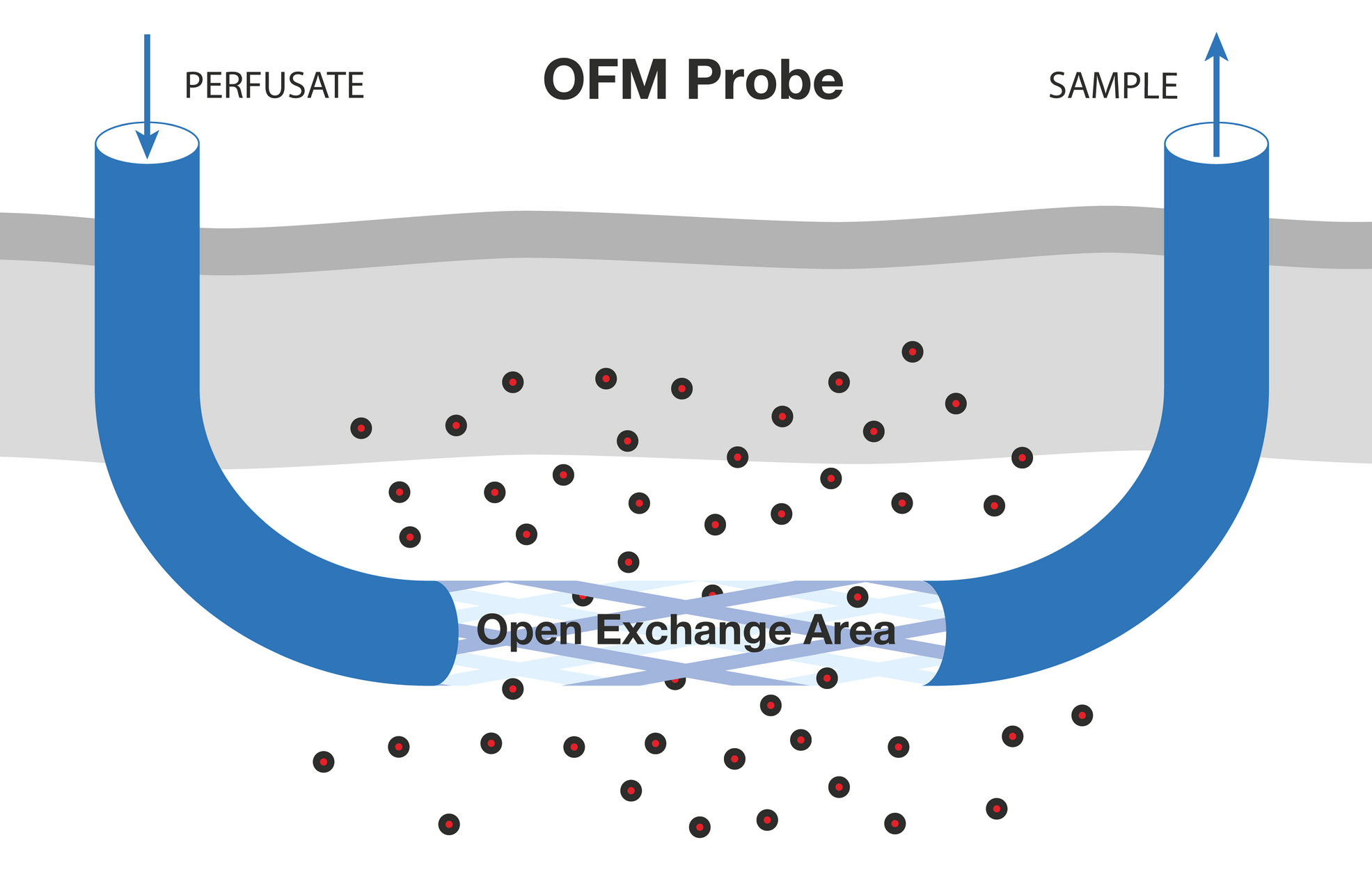
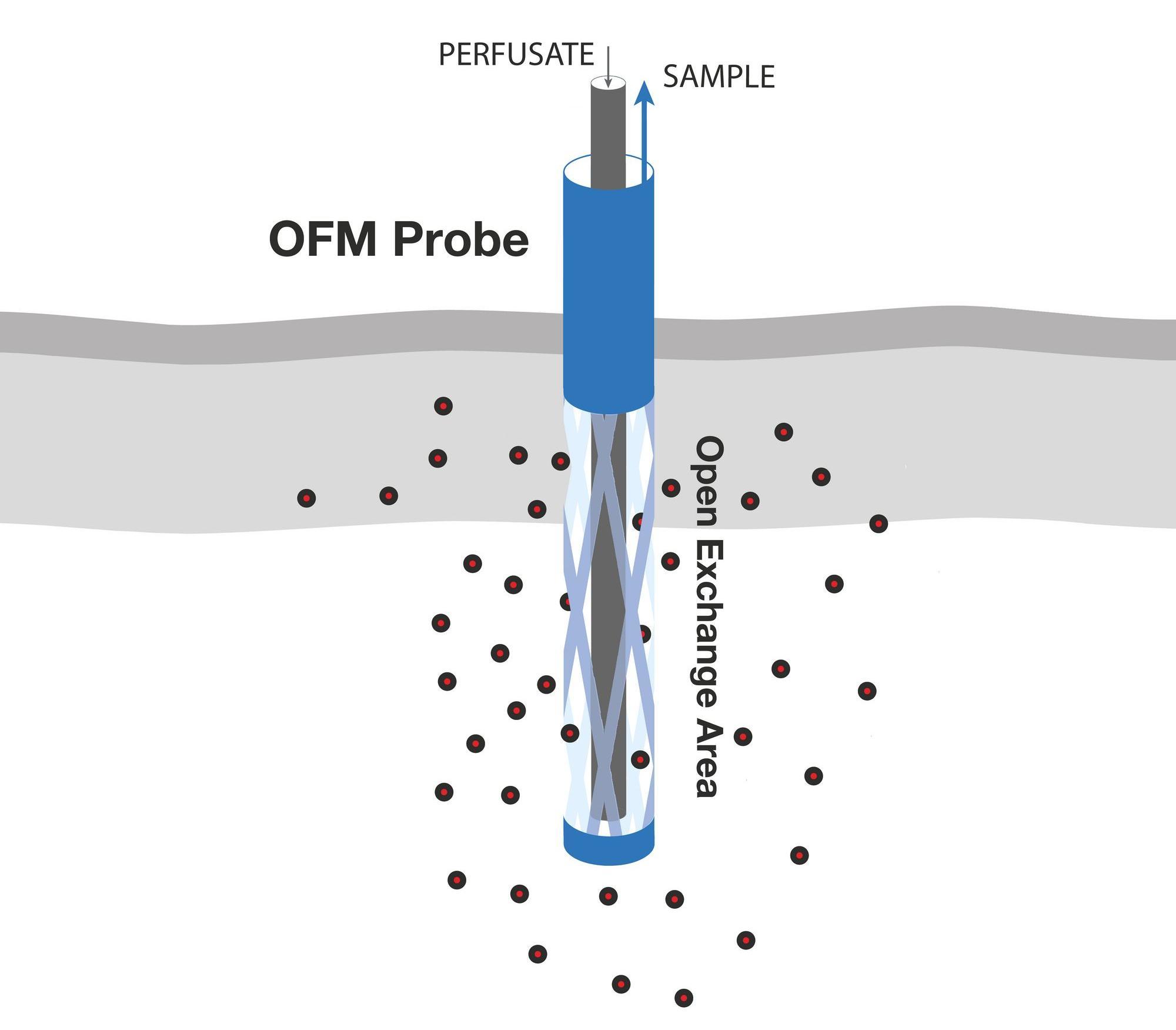
OFM probes are perfused with a physiological solution (perfusate) that is in equilibrium with the interstitial fluid (ISF) of the surrounding tissue. The operating flow rate ranges from 0.1 to 10 µl/min. OFM allows unrestricted exchange of compounds through the open exchange region of the probe. This exchange of compounds between the perfusate of the probe and the surrounding ISF is driven by convection and diffusion and can occur non-selectively in either direction. The cerebral OFM probe (cOFM) works on the same principle as the skin and fat probes. Perfusate is pumped to the tip of the OFM probe through a thin internal tube, then flows out and mixes with interstitial fluid. The mixed fluid (OFM sample) is collected through an external thicker tube. - 1. Minimally invasive, membrane-free probe (PEEK), 0.5 mm diameter
- 2. High-precision stereotactic implantation
- 3. Removable Healing Prosthesis Allows Tissue Regeneration and Re-establishment of Blood-Brain Barrier Integrity After Probe Implantation
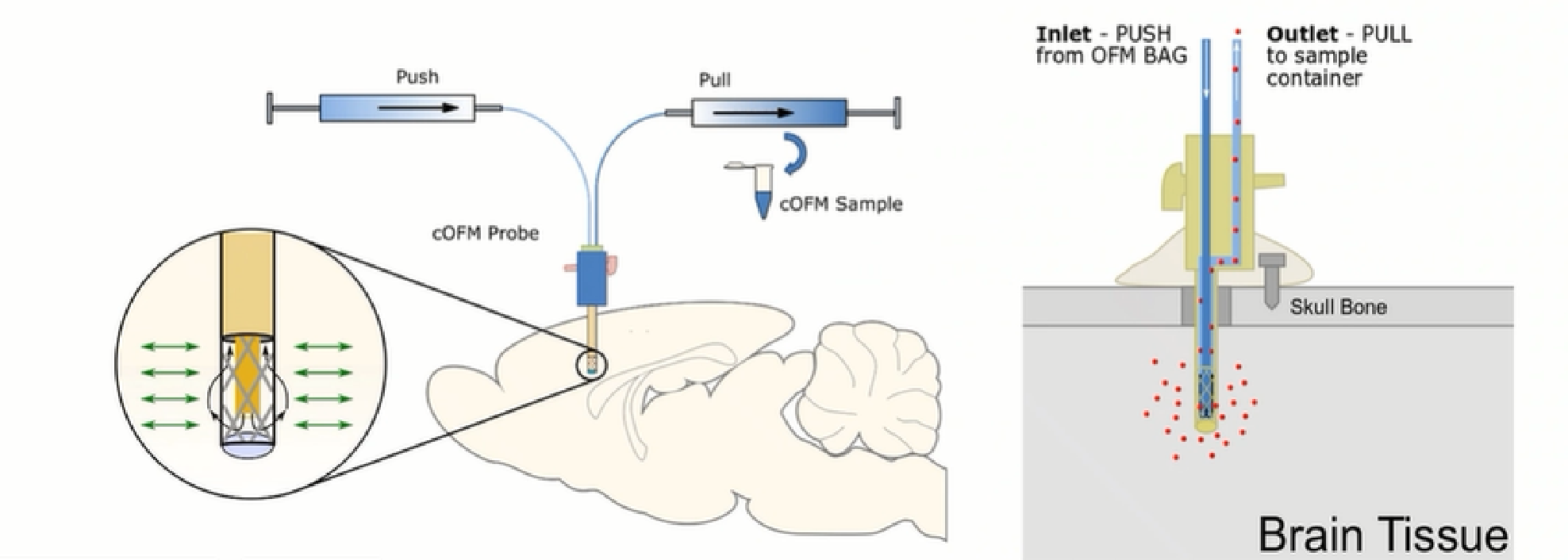
Advantages of OFM
- 1. Continuous sampling of endogenous and exogenous analytes for up to seven days or more
- 2. Higher sampling rates for lipophilic and high molecular weight analytes
- 3. Implantation of the probe directly into the sampling site via BBB (blood-brain barrier) healing prior to sampling
- 4. Minimizing inflammatory responses and interference during sampling
- 5. Minimally invasive sampling methods for rodents and macrofauna
- 6. Membrane-free probes without biological contamination, membrane interactions and clogging
- 7. OFM has been used in mice, rats, pigs, primates and humans
- 8. Probe rod material: PEEK (polyether ether ketone (special polymer materials)) manufactured without any metal, can be used for MRI scans.
- 9. Dual-channel, two independently controllable pump heads that can be used simultaneously to set different perfusion rates; up to eight channels (4 channels per pump head).
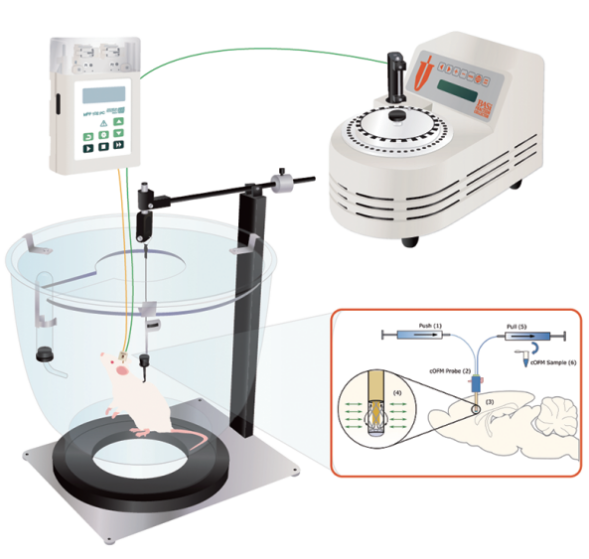
- 10. For rodent studies, the cOFM (Continuous On-line Microdialysis) can also be used in conjunction with the BASi Raturn and Fraction Collectors for continuous ISF (interstitial fluid) sampling.
Our Technology - Open Flow Microperfusion (OFM)
is suitable for different tissues:
COFM brain tissue:
- 1. COFM samples all substances in the interstitial fluid, independent of size, lipophilicity or protein binding.
- 2. COFM sampled brain fluid with minimal tissue reaction and an intact blood-brain barrier.
- 3. COFM is membrane-free and all substances extracted from tissue fluid are independent of size, lipophilicity or protein binding.
- 4. COFMs can be sampled continuously for up to several days, with implantation periods of up to several weeks.
- 5. The COFM research program can be easily translated from the current MD program with only minor adjustments to the timeline.
1.Guidedintubation 2.Healinginserts (dummy probes) 3. Lock bolt A.Samplingprobes 4. Rubber ring B. Protective cover Depending on the size of the brain region of interest, multiple probes can be placed side by side
Open cerebral microperfusion
Tissue response after probe implantation
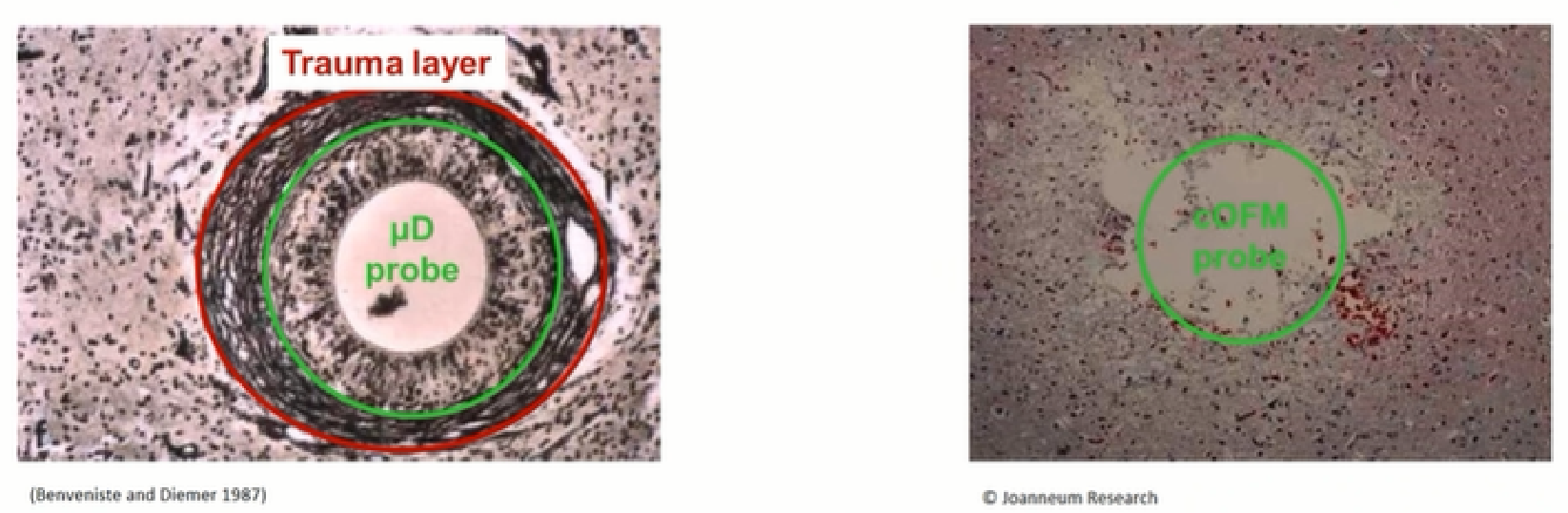
Prolonged implantation of MD probes with peripheral gelatinous scarring (30-60 days) No peripheral gelatinous scar formation after 30 days of cOFM implantation
Birngruber T, Ghosh A, Hochmeister S, Asslaber M, Kroath T, Pieber TR, Sinner F. Long-term implanted cOFM probe causes minimal tissue reaction in the brain. PLoS One. 2014 Mar 12;9(3):e90221. doi: 10.1371/journal.pone.0090221. PMID: 24621608; PMCID: PMC3951198.
Less tissue reaction
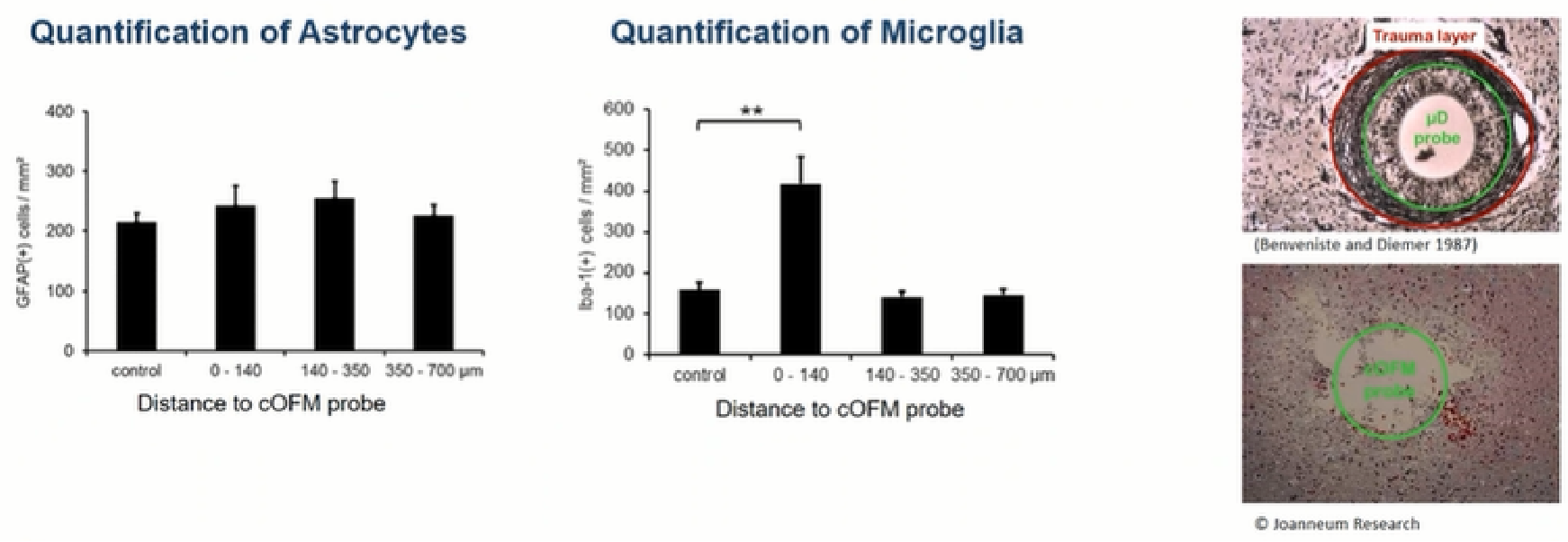
Note: Astrocytes are primarily responsible for glial scar formation and probe envelopes. Microglia are the second most important cell type indicative of glial scar formation.
Birngruber T, Ghosh A, Hochmeister S, Asslaber M, Kroath T, Pieber TR, Sinner F. Long-term implanted cOFM probe causes minimal tissue reaction in the brain. PLoS One. 2014 Mar 12;9(3):e90221. doi: 10.1371/journal.pone.0090221. PMID: 24621608; PMCID: PMC3951198.
Time-resolved substance concentration monitoring
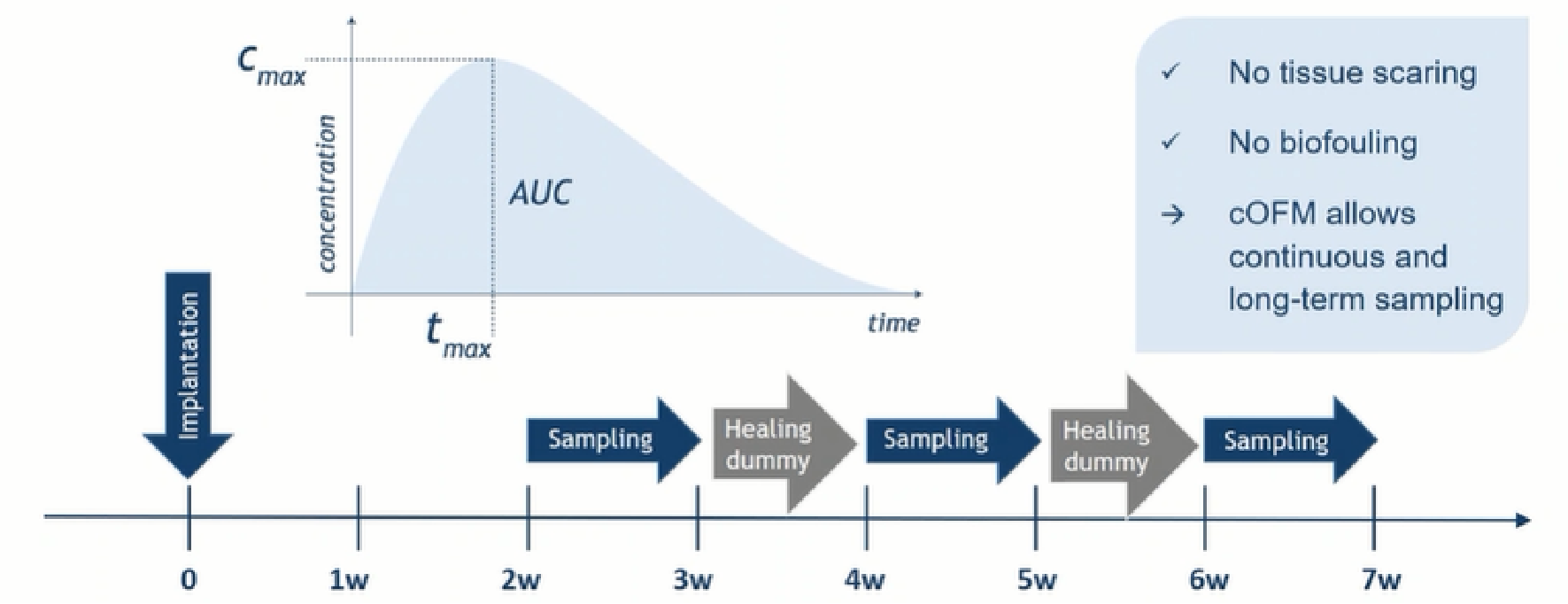
Continuous sampling for 7 days and intermittent sampling for 4 weeks allows for the observation of continuous changes in target substance concentrations over time. Reduces biological contamination and extends sampling time due to membrane-free design
Product inquiry
Looking forward to your message !








